 By Meghan Gabriel, Tricia Lee Rolle, and Chelsea Richwine, ONC
By Meghan Gabriel, Tricia Lee Rolle, and Chelsea Richwine, ONC
LinkedIn: Tricia Lee R.
LinkedIn: Chelsea Richwine
LinkedIn: ONC
The electronic prescribing (e-prescribing) landscape continues to evolve and many positive advances have been made over the past decade. This blog post showcases the rapid growth in e-prescribing, steps taken towards laying the essential groundwork for the current state of e-prescribing, and what remains to be accomplished. This post is our third in the “A Decade of Data Examined” blog series.
Laying the foundation
E-Prescribing
E-Prescribing involves prescribers electronically entering and transmitting prescription information to pharmacies through standards-based software, enhancing convenience, cost-effectiveness, and safety by reducing errors (like handwriting) and automating drug interaction checks. In the early days of e-prescribing, only a few prescribers had stand-alone e-prescribing systems; prescribers primarily relied on manual, paper-based methods for prescribing medications. Since then, federal policy, federal incentives, and state-level action have helped drive the adoption and subsequent use of e-prescribing.
- In 2003, Congress passed the Medicare Modernization Act and in 2006, all states enacted laws to allow e-prescribing of most legend drugs (i.e., drugs that require a prescription).
- In 2008, the Medicare Improvements for Patients and Providers Act (MIPPA) authorized e-prescribing incentives for specific providers.
- In 2009, the Health Information Technology for Economic and Clinical Health (HITECH) Act contributed to the acceleration of e-prescribing by spurring the adoption and use of e-prescribing through certified Health IT, including electronic health record (EHR) systems.
- The Drug Enforcement Administration’s 2010 rulemaking to permit electronic prescribing of controlled substances.
Now, most prescribers have e-prescribing capability integrated in EHRs. Virtually all pharmacies can accept e-prescriptions and 92% of prescribers e-prescribe, an 85 percentage-point increase from 2008 when only 7% of prescribers were sending e-prescriptions (Figure 1).
Figure 1: E-Prescribing Over the Years
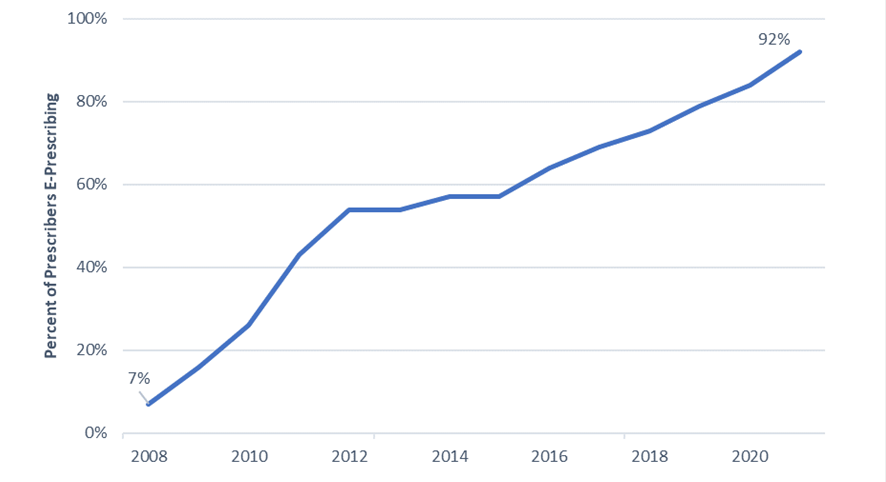
Sources: ONC Analyses 2008-2014 and Surescripts National Progress Reports 2015-2021
E-Prescribing of Controlled Substances (EPCS)
Electronic prescribing of controlled substances (EPCS) nationwide has helped address the opioid epidemic and improve patient outcomes. EPCS can help populate and be integrated with prescription drug monitoring programs (PDMPs) to help curb doctor shopping or overprescribing, which have the potential for patient harm and increased health care costs. The Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act of 2018 further bolstered the use of e-prescribing by mandating use of EPCS by Medicare Part D providers. This helps support patient safety, medication adherence, workflow efficiencies, fraud deterrence, and burden reduction. In addition to this federal mandate, all but 15 states have a current or future EPCS mandate that applies to EPCS more broadly.
Policy efforts to promote widespread EPCS adoption have allowed real-time access to patient information to facilitate better-informed patient care decisions and has resulted in most prescribers (82%) and nearly all pharmacies being EPCS-enabled. Furthermore, there has been an increase among office-based physicians in both adoption and use of EPCS and checking the PDMP. A recent survey showed 87% of office-based physicians who reported checking their state’s PDMP noted associated benefits, such as reducing or eliminating controlled substance prescriptions for patients or confirming the appropriateness of treatments.
Looking Ahead
The nation has made significant strides in establishing electronic methods for safe and secure prescribing, but there is still work to be done to ensure e-prescribing continues to improve patient care and health outcomes. ONC’s Health IT Certification Program and support for standards underpin the e-prescribing’s progress. Additional functionalities that support patient safety and cost reductions such as prescription completeness and delivery of real-time benefit information are on the horizon.
Adding information about why a medication is prescribed enhances the completeness of prescriptions. Currently, e-prescriptions are required to have medication details, including the dosage, strength, quantity, and route of administration. However, most e-prescriptions do not include the indication or reason for the medication, potentially limiting the ability of pharmacists to provide more personalized care for their patients. The goal is for e-prescribing to be more than a tool for sharing prescription info and one that can aid the coordinated care needed for comprehensive medication management and error prevention.
There are also needs to further advance e-prescribing to better support bi-directional access, patient privacy and security, and mitigate data gaps regarding prescription services. The Health Information Technology Advisory Committee (HITAC) through its Taskforce on Pharmacy Interoperability and Emerging Therapeutics recently submitted recommendations including the electronic sharing of prescription information and associated assessment notes to determine prior authorization requirements at the point of care, supporting advanced interoperability to ensure coverage terms are available in real-time, and exploring comprehensive privacy policies to maximize data sharing where authorized.
Congress also reinforced the importance of these capabilities and recently passed an act requiring certified health IT to include a real-time benefit tool (RTBT) that can convey patient-specific cost and coverage information about prescription drugs. This includes real-time access to a patient’s prescription coverage information that will help prescribers make timely and informed decisions about patient care.
Furthermore, ONC has proposed a new rule, Health Data, Technology, and Interoperability: Patient Engagement, Information Sharing, and Public Health Interoperability (HTI-2) that includes provisions to convey patient-specific cost and coverage information through RTBT and include indication on e-prescriptions to continue to promote safe and effective patient care.
Over the past decade, there has been a massive uptake and transformation in e-prescribing. As we look ahead to the next decade, there are ongoing efforts to further enhance e-prescribing to meet the needs of both patients and prescribers. Our current accomplishments and future steps are crucial as we strive for nationwide interoperability, fostering a system where patient data can flow safely and seamlessly, and laying the groundwork for future innovations.
This article was originally published on the Health IT Buzz and is syndicated here with permission.
