 By Mike Berry, ONC
By Mike Berry, ONC
Twitter: @ONC_HealthIT
The Health Information Technology Advisory Committee (HITAC) recently submitted their final report and recommendations related to ONC adopted standards and implementation specifications referenced in federal regulations. The report and recommendations are based on the work of the HITAC’s 2022 Adopted Standards Task Force and are responsive to a 21st Century Cures Act (Cures Act) (42 U.S. Code § 300jj–13) provision that requires the national coordinator to convene stakeholders to review the existing set of adopted standards and implementation specifications and make recommendations with respect to whether to maintain the use of such standards and implementation specifications; or to phase out such standards and implementation specifications.
The inaugural convening of this task force focused on the Cures Act Final Rule Standards maintained on the ONC Standards Hub.
Consistent with the statute, ONC plans to reconvene the Task Force in 2025, and every three years thereafter, to review adopted standards and implementation specifications. The HITAC assembled a diverse group of subject matter experts to form the Adopted Standards Task Force 2022, including from direct patient care, public health, patient advocacy, health IT development, standards development, and academia. Additionally, other subject matter experts were invited to review specific standards and identify viable alternatives to inform the HITAC’s discussions. The HITAC established a method to record and utilize individual members’ inputs to inform group deliberation and establish consensus for each recommendation for every standard and implementation specification included in the scope of review.
The recommendation for each reviewed standard was grouped into one or more of the following categories:
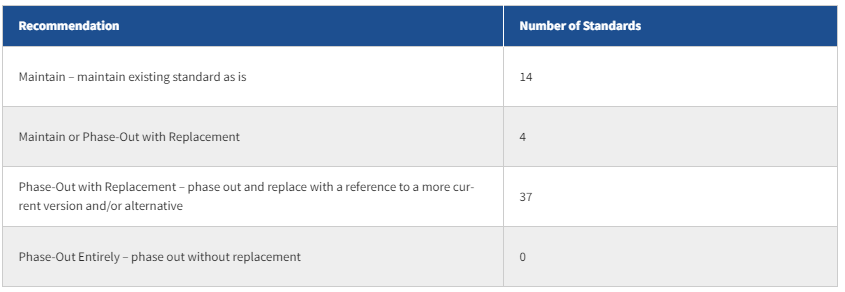
ONC would like to thank the Adopted Standards Task Force, and especially co-chairs Hans Buitendijk and Steven Eichner, for their contributions in developing these HITAC recommendations. ONC will consider the HITAC’s recommendations during future regulatory processes.
The HITAC’s full report is available on HealthIT.gov.
This article was originally published on the Health IT Buzz and is syndicated here with permission.
