 By Steven Posnack, Brett Andriesen and Al Taylor, ONC
By Steven Posnack, Brett Andriesen and Al Taylor, ONC
Twitter: @ONC_HealthIT
Steven’s Twitter: @HealthIT_Policy
The wait is finally over! The ONC Cures Act Final Rule went into effect on June 30, 2020, and since its release one of the most common questions ONC has received is when and how can we submit proposals for new data elements in USCDI? The answer is, now!
The United States Core for Data Interoperability (USCDI) ONC New Data Element and Class (ONDEC) submission system is now open. The ONDEC system gives you the opportunity to submit new data elements and data classes through this ongoing, collaborative process. ONC will accept submissions for USCDI Draft v2 through October 9, 2020. Read more about the process in the USCDI ONDEC Fact Sheet.
The USCDI is a standardized set of health data classes and constituent data elements for nationwide, interoperable health information exchange. It replaces the Common Clinical Data Set that was previously required as part of a number of 2015 Edition health IT certification criteria. You can learn more about the USCDI in the USCDI Fact Sheet. Future versions of the USCDI will be drafted and finalized based on your data element submissions to the ONDEC system.
Once finalized, new versions of the USCDI will also feed into the Standards Version Advancement Process (SVAP). The SVAP allows health IT developers in the ONC Health IT Certification Program to voluntarily update their products to include National Coordinator-approved newer versions of select standards without waiting for future rulemaking. Be on the lookout for the 2020 SVAP comment period starting later this summer.
Here’s how the USCDI ONDEC system works:
- Submit: Stakeholders submit new data elements and data classes in USCDI ONDEC, including information on the use case(s), applicable standards and technical specifications, existing use and exchange of the data, and potential challenges for development and implementation.
- Evaluate: ONC evaluates submissions and assigns each data element to a level (comment, level 1, level 2) that corresponds with the data element’s overall value, maturity, and potential challenges.
- Post: ONC posts submitted data elements on the USCDI page, arranged by level. Submitters can add or change information at a later time to inform a date element’s level determination and other stakeholders can review submissions and contribute to their development through comments and collaboration with submitters and other stakeholders.
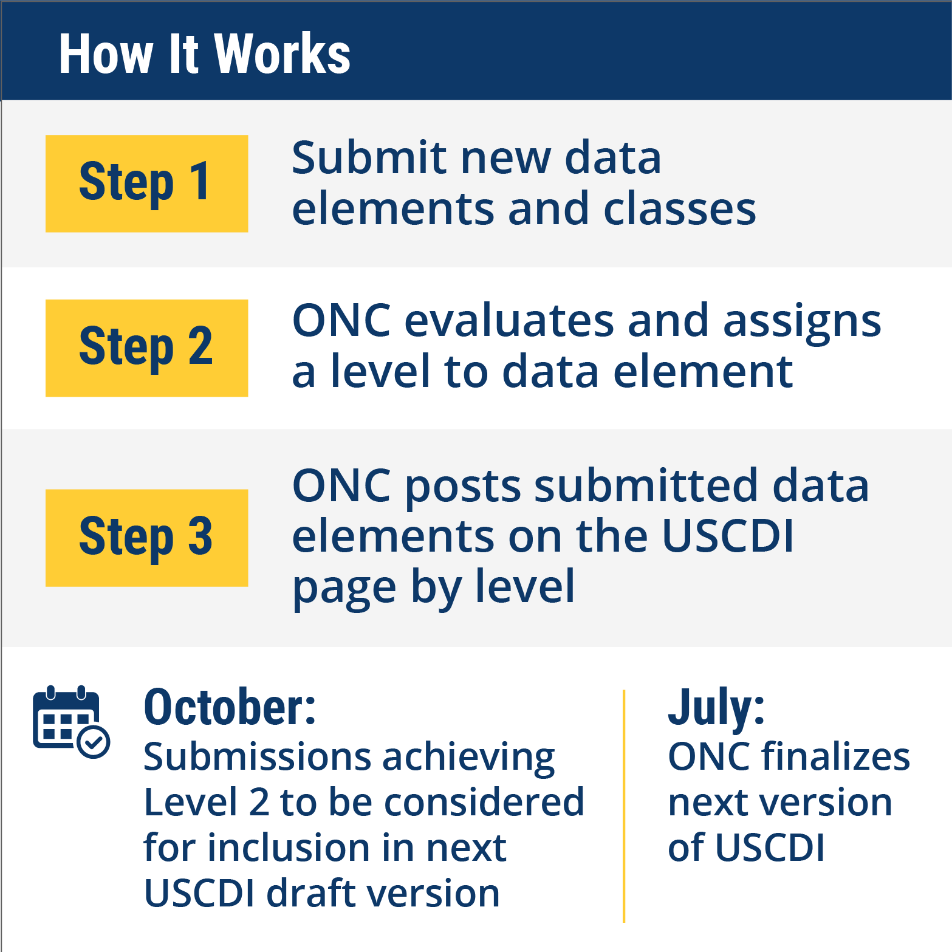
Around October of each year, ONC will consider submissions classified at Level 2 for the next version of the draft USCDI standard. This draft will be presented to the Health IT Advisory Committee (HITAC), and to the public for additional comment before the next version of the USCDI standard is published in July. We will consider submissions to ONDEC received after the October deadline for the following year’s version of the USCDI, as ongoing submissions will be permitted throughout the year.
The USCDI ONDEC system builds upon the success we have achieved engaging with stakeholders through our Interoperability Standards Advisory (ISA) platform and leverages the ISA to allow for USCDI ONDEC submissions and public comments.
To submit new data elements and classes or to make a comment on existing USCDI data elements and classes, an ISA user account is required. You can create a user account by clicking the “Log In” button located at the top right of the ISA page.
We look forward to working with you to advance the USCDI and interoperable exchange of health information!
Note: ONC recently updated the USCDI version 1, which includes a version history and errata noting changes and corrections. The errata address concerns raised by stakeholders on the appropriateness of applicable standards for certain data classes and elements.
This post was originally published on the Health IT Buzz and is syndicated here with permission.
